Introduction:
Expression of PD1 and PD-L1 in MDS patients (pts) is enhanced by treatment with hypomethylating agents (HMA). Pts who are treated with nivolumab (Nivo), an anti-PD1 antibody, are known to show the upregulation of CTLA-4, and conversely, pts who are treated with ipilimumab (Ipi), an anti-CTLA-4 antibody, are known to show the upregulation of PD1 (Garcia-Manero et al ASH 2016). We therefore hypothesized that combination of Nivo and Ipi with azacytidine (Aza) might provide significant efficacy in pts with MDS.
Methods:
This is part of a basket exploratory phase II trial for Nivo and/or Ipi with or without Aza therapy in pts with MDS (NCT02530463). In this analysis, pts from 2 cohorts (Nivo+Ipi in HMA-failure pts, and Aza+Nivo+Ipi in frontline pts) were reviewed. Pts with MDS with age 18 years or older with adequate renal and hepatic function without history of autoimmune diseases were included. In HMA-failure cohort, Nivo was administered at 3mg/kg IV on days 1 and 15 with Ipi 3mg/kg IV on day 1 of a 28-day cycle. In frontline cohort, Aza was administered at 75mg/m2 IV daily on days 1-5 with Nivo 3mg/kg IV on days 6 and 20 with Ipi 3mg/kg IV on day 6 of a 28-day cycle. The study design allowed Aza add-back in Nivo+Ipi cohort after 6 cycles of therapy if there was no response or progression. Response was assessed based on the modified International Working Group 2006 criteria (Cheson et al Blood 2006). Overall response was defined as achieving complete remission (CR), CR with incomplete count recovery (CRi), or hematologic improvement (HI). Overall survival (OS) was calculated from the start date of treatment to the date of death, or last follow-up. Progression-free survival (PFS) was calculated from the start date of treatment to the date of disease progression, or last follow-up. Safety profile events were recorded according to the CTCAE v4.03.
Results:
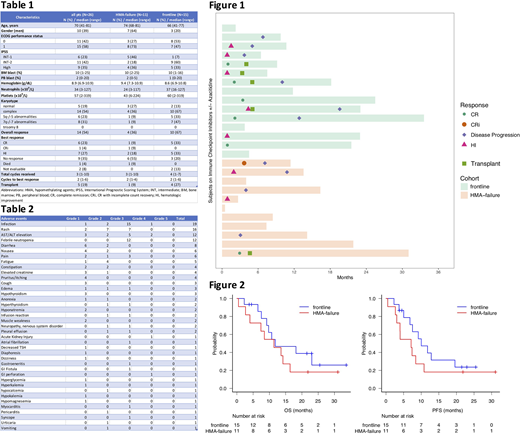
A total 26 pts were treated from 5/2017 to 7/2020 including 11 pts in HMA-failure cohort and 15 pts in frontline cohort. Pts characteristics are summarized in Table 1. The median age was 70 years (range, 41-81), and 39% were male. IPSS risk was INT-1 in 6 (23%) pts, INT-2 in 11 (42%) pts, and high in 9 (35%) pts. Median bone marrow blasts percentage was 10% (range, 1-25). Karyotype was normal in 5 (19%) pts, and complex in 14 (54%) pts. Six (23%) pts had 5/5q abnormalities, 8 (31%) with 7/7q abnormalities. Mutations were detected in TP53 (N=11), TET2 (N=7), DNMT3A (N=7), RUNX1 (N=4), ASXL1 (N=4), PHF6 (N=3), SF3B1 (N=2), U2AF1 (N=2), BCOR (N=2), BCORL1 (N=1), CREBBP (N=1), EZH2 (N=1), JAK1 (N=1), JAK2 (N=1), KDM6A (N=1), NF1 (N=1), PTPN11 (N=1), KRAS (N=1), NRAS (N=1), SRSF2 (N=1), SUZ12 (N=1), WT1 (N=1), and ZRSR2 (N=1). A total 4 (36%) pts in HMA-failure cohort received add-back Aza after 6 cycles of therapy. Overall response rate (ORR) was 36% in HMA-failure cohort including 1 (9%) CR, 1 (9%) CRi, and 2 (18%) HI. In frontline cohort, ORR was 67% including 5 (33%) CR and 5 (33%) HI (Figure 1). With a median follow-up duration of 25 months (mos) (95% CI: 22-NA), 10 (38%) had disease progression and 18 (69%) died. Pts received a median 3 (range, 1-10) cycles with a median 2 (range, 1-6) cycles until achieving best response. Five (19%) pts went to transplant. In HMA-failure cohort, median OS was 11.4 mos (95% CI: 2.7-16.4) and median PFS was 7.1 mos (95% CI: 2.7-10.8). In frontline cohort, median OS was 12 mos (95% CI: 7.5-NA) and median PFS was 10.0 mos (95% CI: 5.0-19.6, Figure 2). Adverse events (AEs) included infection in 19 (73%) pts, rash in 16 (62%), AST/ALT elevation in 12 (46%), febrile neutropenia in 12 (46%), and diarrhea in 8 (31%). Grade 3/4 AEs included infection in 16 (55%) pts, febrile neutropenia in 12 (46%), rash in 7 (24%), and AST/ALT elevation in 7 (24%, Table 2).
Conclusions:
Double immune checkpoint inhibitor blockade with Nivo and Ipi with or without Aza showed clinical activity with tolerable safety profiles in pts with MDS. Longer follow up in larger cohort is needed to further validate the efficacy of this regimen.
Kantarjian:Astex: Research Funding; Immunogen: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Takeda: Honoraria; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; Jazz Pharma: Research Funding; Ariad: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding. Sasaki:Pfizer Japan: Consultancy; Otsuka: Honoraria; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jabbour:Adaptive Biotechnologies: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding. Alvarado:FibroGen: Research Funding; Daiichi-Sankyo: Research Funding; MEI Pharma: Research Funding; Sun Pharma: Research Funding; Tolero Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; BerGenBio ASA: Research Funding. DiNardo:MedImmune: Honoraria; ImmuneOnc: Honoraria; Calithera: Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Syros: Honoraria; Takeda: Honoraria; Jazz: Honoraria; Novartis: Consultancy; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Orsenix: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Borthakur:Treadwell Therapeutics: Consultancy; Incyte: Research Funding; PTC Therapeutics: Research Funding; Novartis: Research Funding; Abbvie: Research Funding; Jannsen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; Polaris: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Curio Science LLC: Consultancy; FTC Therapeutics: Consultancy; Argenx: Consultancy; PTC Therapeutics: Consultancy; BioLine Rx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy. Bose:Astellas Pharmaceuticals: Research Funding; Pfizer, Inc.: Research Funding; Celgene Corporation: Honoraria, Research Funding; NS Pharma: Research Funding; CTI BioPharma: Honoraria, Research Funding; Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Blueprint Medicines Corporation: Honoraria, Research Funding; Constellation Pharmaceuticals: Research Funding; Kartos Therapeutics: Honoraria, Research Funding; Promedior, Inc.: Research Funding. Pemmaraju:Pacylex Pharmaceuticals: Consultancy; DAVA Oncology: Honoraria; AbbVie: Honoraria, Research Funding; SagerStrong Foundation: Other: Grant Support; LFB Biotechnologies: Honoraria; Novartis: Honoraria, Research Funding; Samus Therapeutics: Research Funding; MustangBio: Honoraria; Blueprint Medicines: Honoraria; Plexxikon: Research Funding; Incyte Corporation: Honoraria; Cellectis: Research Funding; Celgene: Honoraria; Roche Diagnostics: Honoraria; Daiichi Sankyo: Research Funding; Affymetrix: Other: Grant Support, Research Funding; Stemline Therapeutics: Honoraria, Research Funding. Cortes:Novartis: Consultancy, Research Funding; Sun Pharma: Research Funding; Takeda: Consultancy, Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Telios: Research Funding; Astellas: Research Funding; Pfizer: Consultancy, Research Funding; Amphivena Therapeutics: Research Funding; Arog: Research Funding; BiolineRx: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Merus: Research Funding; Immunogen: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Kadia:Astra Zeneca: Research Funding; Abbvie: Honoraria, Research Funding; Cyclacel: Research Funding; Ascentage: Research Funding; Amgen: Research Funding; Genentech: Honoraria, Research Funding; Novartis: Honoraria; Pfizer: Honoraria, Research Funding; Celgene: Research Funding; JAZZ: Honoraria, Research Funding; Cellenkos: Research Funding; BMS: Honoraria, Research Funding; Astellas: Research Funding; Incyte: Research Funding; Pulmotec: Research Funding. Konopleva:Cellectis: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Calithera: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Sanofi: Research Funding; Genentech: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Ablynx: Research Funding; Eli Lilly: Research Funding; AstraZeneca: Research Funding; Agios: Research Funding; Ascentage: Research Funding; Amgen: Consultancy; Kisoji: Consultancy. Garcia-Manero:Acceleron Pharmaceuticals: Consultancy, Honoraria; Novartis: Research Funding; Amphivena Therapeutics: Research Funding; Onconova: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; AbbVie: Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy; H3 Biomedicine: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.